Researchers at the Stanford University School of Medicine have discovered the combinations of biological and chemical signals needed to rapidly generate human cell types from human embryonic stem cells, according to Stanford Medicine News. Pure populations of up to 12 cell types can now be created in five to nine days, as opposed to the weeks or months previously required.
The senior authors of the study are Irving Weissman, director of Stanford’s Institute for Stem Cell Biology and Regenerative Medicine and its Ludwig Cancer Center, and Lay Teng Ang of the Genome Institute of Singapore. The lead authors of the study are Stanford Ph.D. student Kyle Loh and research assistant Angela Chen. The study was published on July 14 in Cell.
Cell types that can be produced include bone, heart muscle and cartilage. This may allow researchers to create beating heart cells for heart attack patients or cartilage or bone to aid in joint repair.
“Previously, people had some success in turning stem cells into different cell types,” Loh said. “The problem was, it often took a very long time, like weeks or months, and moreover, they generated an impure mixture of cell types.” Researchers might have gotten bone cells, heart cells and pancreatic cells, as opposed to the pure populations generated by the newfound method.
Loh said, “We want to inject these cells into patients one day, with the goal of regenerating specific human tissue.”
The study also demonstrates patterns of gene expression that occur during human embryo segmentation and confirms that human development relies on evolutionary-conserved processes. This provides insight into how congenital defects occur.
Human embryonic development is relatively difficult to study, as the international ethical standard, the “14-day rule” limits laboratories from cultivating human embryos for longer periods of time.
Embryonic stem cells are pluripotent, which means they can become any cell type in the body. The human embryo has three germ layers: the ectoderm, the endoderm and the mesoderm. Each germ layer gives rise to certain cell types during embryonic development. Loh and Chen focused their research on the mesodermally derived cell types, which include cardiac and skeletal muscle, connective tissue, bone, blood vessels, blood cells, cartilage and parts of the kidney and skin.
Loh and Chen specialized a human embryonic stem cell line by applying specific combinations of signaling molecules.
Their strategy can be thought of as a “yes-and-no” technique. They apply factors that encourages differentiation into one type but also blocks differentiation to another type.
“Let’s say you’re trying to differentiate bone cells,” Chen said. “We’re adding positive signals so that something promotes bone formation and, at the same time, inhibits the pathway at the very beginning [that goes] towards the heart.”
In another example, cells in the primitive streak, present in the early embryo, become either endoderm or a type of mesoderm. To drive the cells to become mesodermal, researchers inhibit the activity of the signaling molecule transforming growth factor beta (TGF-B). Adding a signaling molecule called WNT and blocking the activity of the bone morphogenetic protein (BMP) molecule encourages differentiation into a specific type of mesoderm, and the reversing the function of these molecules drives cells to become the other type of mesoderm.
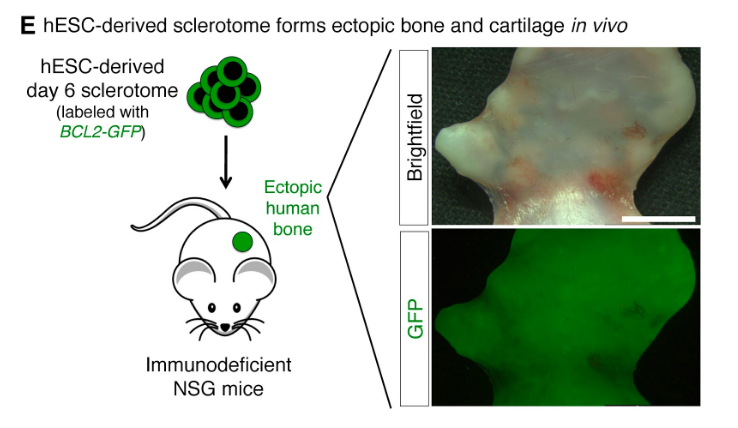
Loh and Chen have generated human bone by transplanting bone cell precursors into laboratory mice. They have also generated beating heart muscle cells and 10 more mesodermal-derived cell lineages.
The ability to create pure populations of human cell types from stem cells is a key step in future regenerative medicine treatments.
“I think it will not directly improve any current treatment, but I think it opens the door to many future ones,” Loh said of the discovery. “Our ability to produce these new cells will be kind of the gateway to making a lot of future therapies work.”
Contact Tanushri Sundar at tanushrisundar ‘at’ gmail.com.