Marc Tessier-Lavigne told shareholders in 2009 that his research would “turn our current understanding of Alzheimer’s upside down.” Now, the former Stanford president and his co-authors have retracted the paper he once heralded, conceding they do not have confidence in its data.
The prominent journal Nature announced the retraction in a Dec. 18 note signed by all four co-authors. The note acknowledged a number of image anomalies and biostatistical errors, but denied that the study had included falsified data. Tessier-Lavigne was first urged to retract the paper over a decade ago but maintained as recently as July that he would not.
“As with all of my papers, at the time of publication of Nature 2009, I believed the results in the paper were correct and accurately presented,” Tessier-Lavigne wrote in a statement to The Daily last week. “I absolutely believe that there are no falsified data in the paper,” he wrote in a subsequent email.
This retraction is Tessier-Lavigne’s fourth in as many months, a stunning turn of events for a researcher of his stature. A wealthy and influential neuroscientist, Tessier-Lavigne served as chief scientific officer at Genentech and president of Rockefeller University before he assumed the presidency of Stanford. He resigned as president this summer after a Stanford-sponsored investigation confirmed a pattern of falsified research emerging from labs he ran.
Tessier-Lavigne has not been accused of manipulating data himself or directly encouraging falsification. But the Stanford investigation found that he failed to correct the scientific record on various occasions when falsification was brought to his attention across three different labs and two decades.
Retractions remain exceedingly rare for scientific papers: Just eight out of every 10,000 are retracted, according to a Retraction Watch database. Two of Tessier-Lavigne’s influential neurodevelopment papers published in Science and a third published in Cell were withdrawn earlier this fall after they were found to contain manipulated images. Another Tessier-Lavigne paper published in Nature was issued an expression of concern over “manipulation of research data” this month, implying it will likely face correction or retraction.
But the 2009 paper, which garnered a towering 816 citations according to Clarivate’s Web of Science, was one of the most significant papers Tessier-Lavigne had published. It claimed to have found the cause of Alzheimer’s and suggested a potential course for treating the deadly disease.
As early as 2008, the year before the paper was published, experiments conducted at Genentech suggested that its central finding, a binding between two specific proteins, was at best unreliable.
“Prior to publication of the paper, employees other than the authors performed binding experiments that showed inconsistent results,” Genentech said in an April statement. “Senior leaders at Genentech including Dr. Tessier-Lavigne knew of the inconsistent binding results,” it continued in the next paragraph. Evidence that the binding was inconsistent was not included in the paper nor publicly mentioned by Tessier-Lavigne.
Tessier-Lavigne told Stanford investigators that “despite being the Principal Investigator, he had never previously been provided with the full set of inconsistent binding results and, had he known their extent, he would have engaged in additional investigative experimentation.” In a statement last week, he reiterated that he had not been aware prior to publication that the central binding in his research could not be reliably replicated. Genentech declined to elaborate on its readout.
Despite the internal uncertainty, Genentech’s public rollout lauded the study as a long-awaited breakthrough in combating Alzheimer’s. “Because of this research,” Genentech’s 2009 annual letter to shareholders read, the company was working to create novel treatments that would “help the millions of people who currently suffer from this devastating disease.” Within Genentech, there was speculation that the research could win a Nobel Prize, and Tessier-Lavigne went on a media tour to promote the paper.

Top executives, including Tessier-Lavigne, also used the paper as part of a campaign to raise the purchase price that pharmaceutical giant Roche would pay to acquire Genentech, a negotiation ongoing at the time. According to a transcript of a March 2009 shareholder presentation, Tessier-Lavigne made the case that “when we decide to enter an area, we enter in full force with the aim of making a difference very rapidly.” His research, he said, was an example of that.
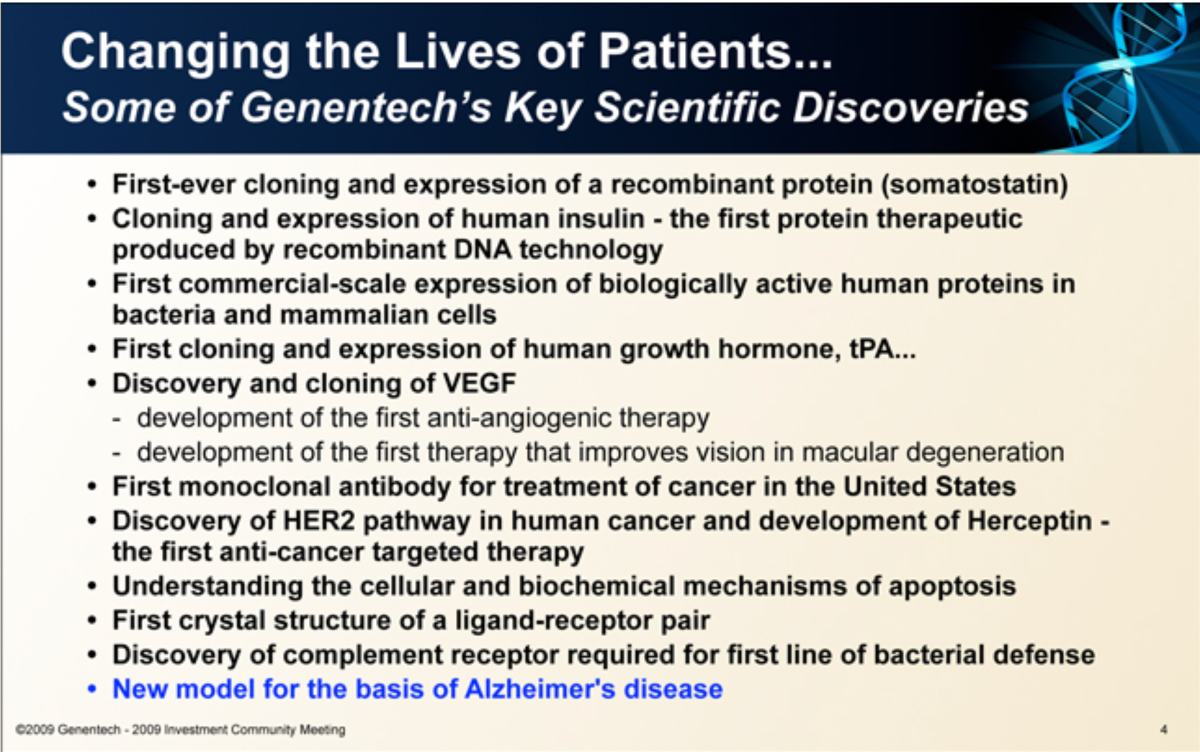
On a slide listing the company’s “key scientific discoveries,” only this research appeared highlighted in blue.
Genentech’s campaign successfully convinced investors that the biotech company was undervalued. Roche’s offer increased from $86.50 to $95 a share, a difference of roughly $4 billion. It remains unclear exactly how much the now-retracted Alzheimer’s paper factored into Roche’s calculus; one high-ranking Genentech executive told The Daily that “the information circle was small,” though sticking points were said to include Avastin, a cancer drug going through a trial at the time, according to another senior figure at Genentech.
Genentech declined to comment last week on negotiations with Roche.
By 2012, it had become clear to researchers both inside Genentech and at rival pharmaceutical companies that the research in the Tessier-Lavigne paper was not reproducible. Genentech’s Research Review Committee, a group of top-level executives at the company, authorized attempts to reassess the program and ultimately decided to cease further research.
According to eight prominent researchers and executives with knowledge of the review, executives at the company were convinced that the research had been based on falsified data. The “rising star” researcher who had spearheaded the study, Anatoly Nikolaev, abruptly left the field of bioscience to attend community college in Michigan. Tessier-Lavigne, Genentech and Nikolaev deny that there was ever discussion of fraud in the paper.
Tessier-Lavigne was urged to retract the paper amid the 2012 review, Genentech confirmed in April. Sources The Daily interviewed said at least four senior figures within the company had urged withdrawal. Tessier-Lavigne chose not to, instead publishing subsequent papers that walked back several of the claims. He continued to cite the paper in grant applications, according to National Institutes of Health filings reviewed by The Daily. Tessier-Lavigne declined to answer questions last week about why he did not retract the paper in 2012.
The Stanford-sponsored report on Tessier-Lavigne’s research stated in mid-July that “allegations of fraud related to the paper appear to be mistaken.” Investigators speculated that a separate instance in 2010 of research misconduct in Tessier-Lavigne’s lab had been conflated with the 2009 study. Genentech insiders who spoke to The Daily denied that they had confused the two, and identified three separate episodes of alleged research misconduct in Tessier-Lavigne’s lab. The Stanford report did not address the third episode, which was brought to the University’s attention in correspondence obtained by The Daily in March.
It has since emerged that key sources declined to speak to Stanford’s investigators because they were not promised anonymity despite non-disclosure agreements.
Despite not finding fraud, the Stanford investigation concluded the 2009 paper “fell below accepted scientific practices, let alone Dr. Tessier-Lavigne’s self-described standard of scientific excellence.”
At the time the Stanford report was released, Tessier-Lavigne acknowledged flaws but did not intend to retract the paper. On a public lab website at that time, he wrote that he wanted to make clear to Nature readers that aspects of the paper had not held up and that certain data were unreliable. “I intend to issue such a correction as soon as possible,” he wrote.
It is unclear exactly what led him to retract the paper entirely rather than correcting only certain parts. Editors at Nature did not respond to a request for comment and Tessier-Lavigne said only that the decision was “based on our assessment of how to proceed given the new anomalies that only came to light this past year,” referring to duplicate images in the study.
Stephen Neal, the chairman emeritus of Cooley LLP who has served as Tessier-Lavigne’s lawyer, wrote in February that “Dr. Tessier-Lavigne’s later papers did not repudiate the Paper’s primary findings and a correction or retraction of those findings would have been unwarranted and inappropriate.” Neal also wrote that “the Paper’s original results were accurately reported.”
But in this week’s retraction note, Tessier-Lavigne and the other authors acknowledged that “our subsequent research showed that certain specific claims in the original article were not correct.” The paper’s central theory, about a protein bind that the authors said was hijacked by Alzheimer’s and triggered a process that caused neurons to prune themselves, was inaccurate in several ways.
The retraction notice also acknowledged that four panels appeared to have been reused to represent different experiments, and a fifth panel appeared to contain a blot partially duplicated from a sixth panel. There were also unspecified errors in “certain biostatistical calculations underlying some figures,” the notice said.
Concerns about Tessier-Lavigne’s research first emerged in 2015 on a scientific forum called PubPeer. They were resurfaced in a Daily report last year that addressed four Tessier-Lavigne papers, though the 2009 Nature paper only came under public scrutiny after a Feb. 17 Daily article detailed the recollections of four senior scientists and executives at Genentech.
According to Gene Sykes, chair of the search committee to find a permanent replacement for Tessier-Lavigne, the committee has “plans to do due diligence in a way that it was not done in the previous search.” Stanford has declined to specify those plans.
