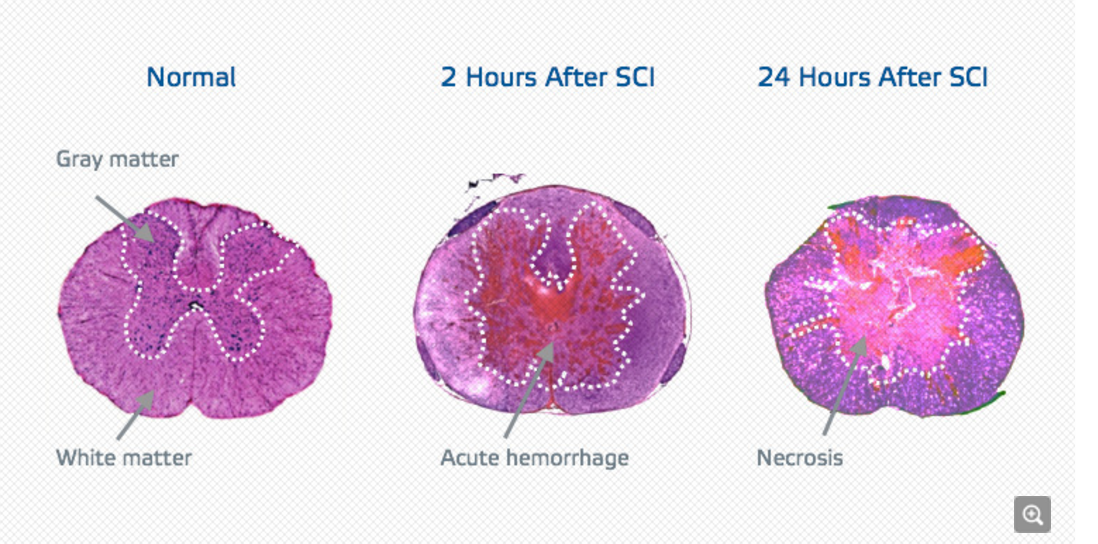
On July 12, InVivo Therapeutics Holdings Corporation named Stanford Hospital as one of 34 testing sites for its neuro-spinal scaffold study. The study’s implementation of a biodegradable implant will support remaining spinal tissue and promote healing in patients with spinal cord trauma.
Stanford director of neurosurgical oncology and clinical assistant professor of neurosurgery Atman Desai was named the principal investigator. Desai’s multiple focuses in spinal surgery include minimally invasive and robotic techniques.
“We look forward to working with Stanford Medicine and Dr. Desai, especially given Dr. Desai’s expertise in spinal cord injury treatment,” said InVivo CEO and Chairman Mark Perrin in their press release.
The INSPIRE Study, short for the InVivo Study of Probable Benefit of the Neuro-Spinal Scaffold for Safety and Neurologic Recovery in Subjects with Complete Thoracic AIS A Spinal Cord Injury, is designed to assist patients with a complete spinal cord injury: when the patient experiences neither sensory nor motor function beneath the site of trauma. The wounds are classified as “AIS A” by the International Standards for Neurological Classification of Spinal Cord Injury.
The scaffold provides three-dimensional cellular support, as opposed to the two-dimensional support such as suturing. It is made of two polymers in a porous cylinder and customized for the patient’s injuries. Scaffolds are designed to last a few weeks before resorption.
The study is undergoing clinical trial and expected to conclude in 2027.
Contact Jessica Jen at jessicajen23 ‘at’ gmail.com