The Food and Drug Administration (FDA) approved the gene therapy Casgevy in December, which uses CRISPR/Cas9 genome editing technology to treat sickle-cell disease (SCD) in patients twelve years and older. The drug, also called “exa-cel,” is the first gene-editing medical treatment to be FDA-approved.
Two Stanford faculty were closely involved with the process: Stanford Professor of Pediatrics Matthew Porteus M.D. Ph.D ’94 pioneered a method of safely delivering CRISPR/Cas9 into blood stem cells, an integral step in the Casgevy treatment process. Joseph Wu, the director of the Stanford Cardiovascular Institute and a professor of medicine and radiology at Stanford Medicine, sat on the FDA advisory committee that recommended the drug’s approval.
The CRISPR/Cas9 gene-editing system is based on a bacterial immune response against viruses. CRISPR/Cas9 technology utilizes a human-engineered guide RNA to lead a scissors-like enzyme called Cas9 to cut a specified region of DNA.
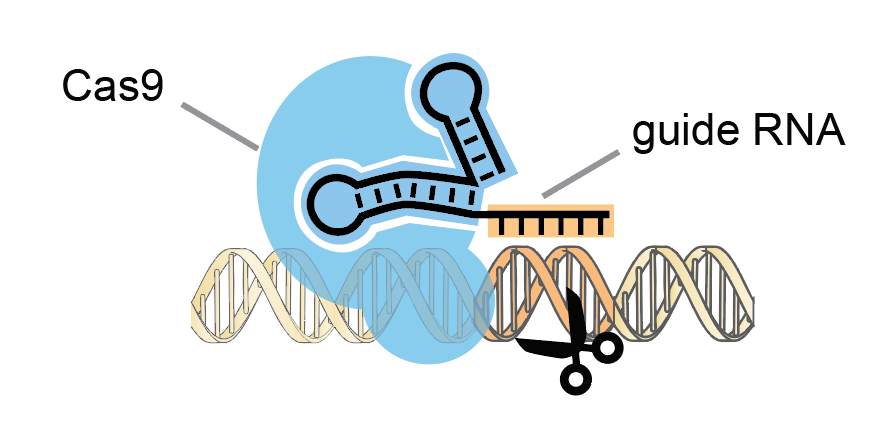
In 2020, biochemists Jennifer Doudna and Emmanuelle Charpentier won the Nobel Prize in Chemistry based on prior work developing a method for human genome editing using the CRISPR/Cas9 system. Stanley Qi, Associate Professor of Bioengineering at Stanford, collaborated with Doudna at U.C. Berkeley as a Ph.D. student.
He described the technology as a mechanism for “genome surgery” that can precisely cut a DNA region then “operate” at that point to deactivate or activate genes.
SCD is the first genetic disease to be treated with an FDA-approved gene-editing drug. The disease is one of the most common serious genetic conditions, affecting about 100,000 Americans each year and predominantly impacting individuals with African ancestry. However, drug development has historically overlooked the disease.
Patients with SCD produce defective hemoglobin, a protein found in red blood cells that normally delivers oxygen to organs and tissues. Flawed sickle cell hemoglobin causes red blood cells to disfigure into a “sickle” c-shape. Sickled red blood cells stick together and restrict the flow of oxygenated blood in blood vessels, causing extreme pain, organ destruction and shortened patient lifespans.
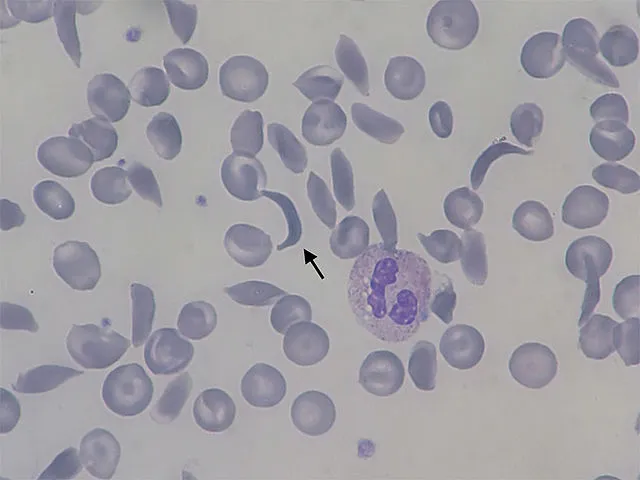
Casgevy extracts patient blood stem cells and then uses a Cas9 molecule to deactivate a gene involved in turning off fetal hemoglobin production. This process reactivates fetal hemoglobin production to combat the production of defective adult hemoglobin in patients with SCD. Genetically modified stem cells are later returned to patients, prompting the release of newly-produced fetal hemoglobin which can block red blood cells from twisting into the sickle shape.
The results of the drug’s clinical trial showed that 93.5% of patients who received sufficient follow up did not suffer severe pain or organ damage events for twelve consecutive months.
The FDA’s decision to approve Casgevy was influenced by an advisory committee that met in October to discuss the merits and risks associated with the drug’s clinical implementation.
“This FDA approval will open up an avenue for future genetic-based treatment of other diseases that can afflict our patients,” wrote Wu, who sat on the FDA advisory board.
Despite Casgevy’s FDA approval, concerns about the drug’s cost, accessibility and safety linger.
Jamie Imam Ph.D. ’12, a Biology lecturer and director of the Biology honors program at Stanford, described herself as “cautiously optimistic” about Casgevy.
“It will be important to see longer-term safety data, and it will also be critical to see if this one-time treatment will be accessible to the people who need it,” Imam wrote.
Even though Casgevy eliminates the need for a bone marrow transplant, currently considered the only cure for SCD, the treatment shares similar challenges. Casgevy’s current price is $2.2 million per patient, which has raised concern due to the underserved demographics predominantly impacted by SCD. Only nine US treatment centers are authorized to deliver the treatment, which lasts one year and involves chemotherapy.
According to Qi, another concern is that Casgevy may permanently and irreversibly modify a patient’s genetic code at incorrect locations.
“The big solution for future drugs is to not alter the DNA codes themselves, but the epigenetics,” Qi said.
Epigenetics refers to the chemistry of DNA that controls gene expression without changing genetic codes. Chemical modifications can make DNA more tightly packed or loosened up, potentially increasing or decreasing gene expression.
Other Stanford research focuses on alternative gene-editing methods to treat SCD. A CRISPR Therapeutics Scientific Founder, Porteus helped recommend SCD as an initial focus targeted by the first CRISPR/Cas9 drug. He and his lab continue to develop Kamau, a different gene-editing drug that directly corrects the SCD mutation to convert sickle cell hemoglobin into healthy adult hemoglobin.
“We cut and fix, rather than cut and turn on fetal hemoglobin,” Porteus said.
Casgevy was not the sole gene therapy approved to treat SCD in December. The FDA also approved Lyfgenia, which adds a corrective gene to produce functional adult hemoglobin. On Jan. 16, the FDA further extended approval of Casgevy to treat transfusion-dependent beta-thalassemia, another hereditary blood condition in which patients struggle to produce sufficient hemoglobin and rely on frequent red blood cell transfusions to survive.
In light of Casgevy’s FDA approval, Qi emphasized the importance of understanding how the CRISPR/Cas9 system works. “Someday, maybe you or someone you know will receive these new drugs.”